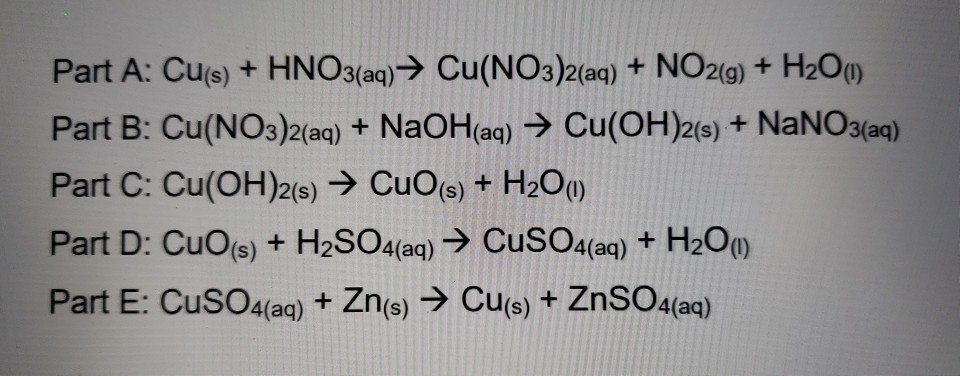
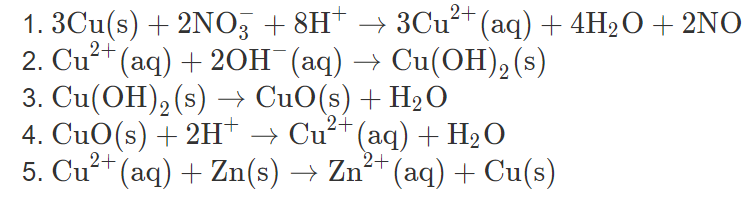Rotating flow of Ag-CuO/H2O hybrid nanofluid with radiation and partial slip boundary effects | SpringerLink

Temperature contour with CuO-H2O (DI) as a working fluid in HCE at 40 LPH. | Download Scientific Diagram
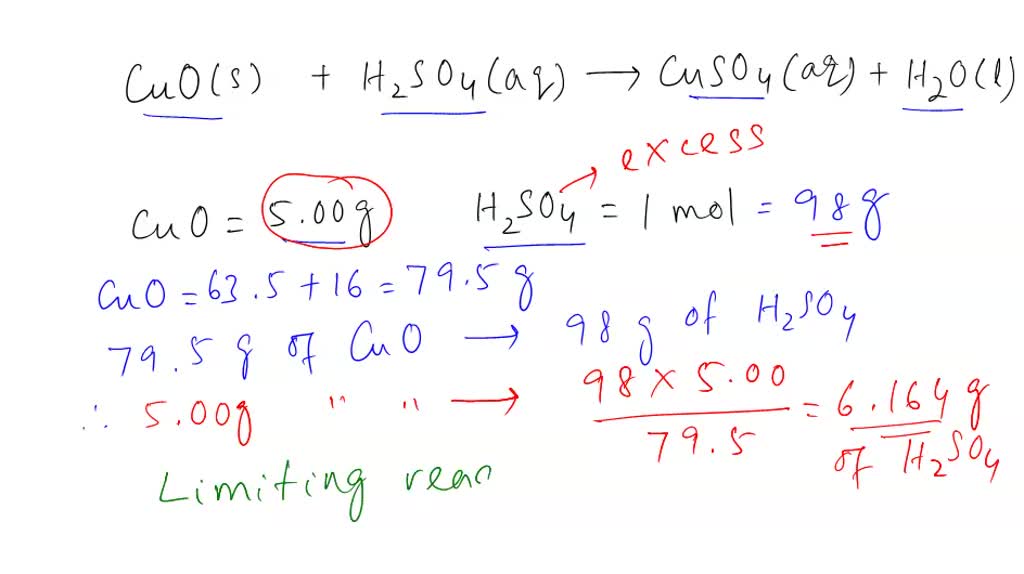
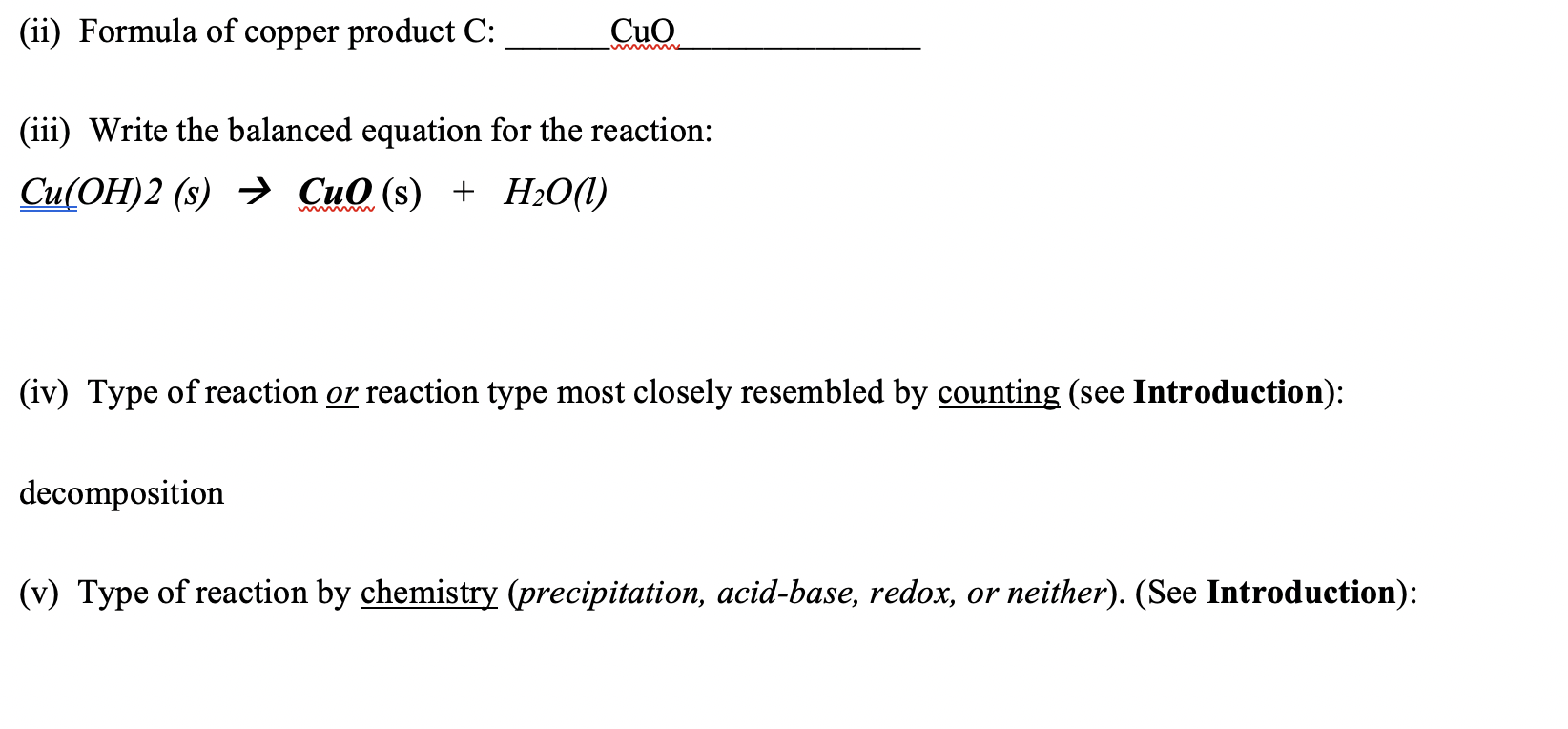
SOLVED: CuO (s) + H2SO4 (aq) → CuSO4 (aq) + H2O (l) When 5.00 g of copper oxide reacts with 1.00 mole of H2SO4, which of the following statements is(are) TRUE? Assume

Frontiers | Thermal efficiency in hybrid (Al2O3-CuO/H2O) and tri-hybrid (Al2O3-CuO-Cu/H2O) nanofluids between converging/diverging channel with viscous dissipation function: Numerical analysis

Pushing the Limits of Rapid Anodic Growth of CuO/Cu(OH)2 Nanoneedles on Cu for the Methanol Oxidation Reaction: Anodization pH Is the Game Changer | ACS Applied Energy Materials

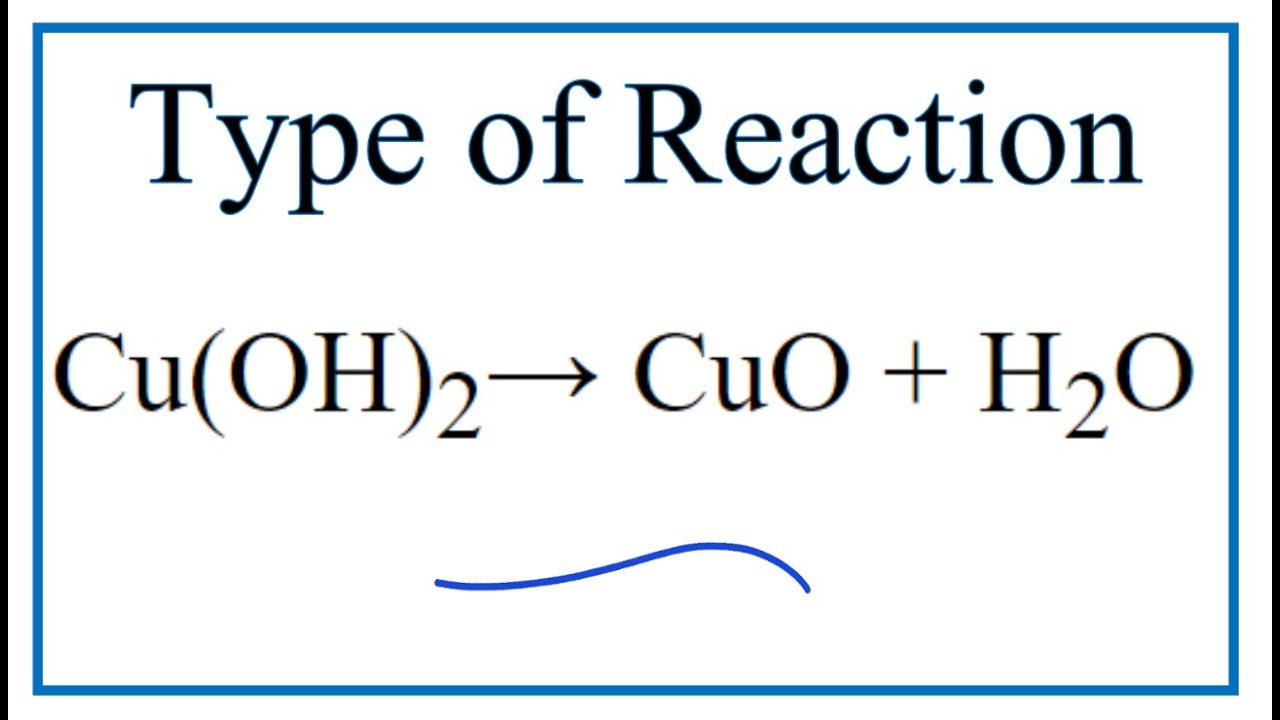
Given the redox reaction: CuO (s) + H2(g)harr Cu(s) + H2O(g) i) Identify the species which undergo reduction and which undergo oxidation